Definition of Cosmetics
Under China's Cosmetic Supervision and Administration Regulations (CSAR), cosmetics are defined as daily-use chemical products applied to the human body surface, such as skin, hair, nails, or lips, by smearing, spraying, or other similar methods, for the purpose of cleansing, protecting, beautifying, or altering appearance.
Classification of Cosmetics
China classifies cosmetics into two main categories:
- Special Cosmetics: products with specific functions, including hair dyeing, perming, anti-freckle/ whitening, sunscreen, anti-hair loss products, and cosmetics claiming new efficacies.
- Ordinary Cosmetics: all other cosmetics, including general skin and hair care, body care, nail care, makeup, deodorants, perfumes, and toothpaste (which is now regulated as an ordinary cosmetic).
Note: Products intended for hair growth, breast enlargement, or body sculpting are not classified as cosmetics under CSAR.
Market Access Requirements
Except for cosmetics sold via cross-border e-commerce (which are currently exempt from registration), all cosmetics (excluding ordinary beauty soaps) must undergo filing or registration before being placed on the Chinese market.
- Special cosmetics require registration with the National Medical Products Administration (NMPA).
- Ordinary cosmetics require filing with the provincial-level MPAs.
Qualifications of Registrants and Filers
Registrants or filers are responsible for the quality, safety, and efficacy claims of their cosmetics. They must:
- Be a legally established enterprise or other organization;
- Maintain a quality management system suited to their cosmetics; and
- Have the capacity for adverse reaction monitoring and evaluation.
Role of the Domestic Responsible Person (DRP)
Foreign companies must appoint a domestic responsible person (DRP) to manage registration or filing. The DPR must:
- Submit applications on behalf of the registrant and filer;
- Assist with adverse reaction monitoring and evaluations;
- Support recalls and post-market safety management;
- Share legal responsibility for product safety and quality in China; and
- Cooperate with inspections by regulatory authorities.
Application Process
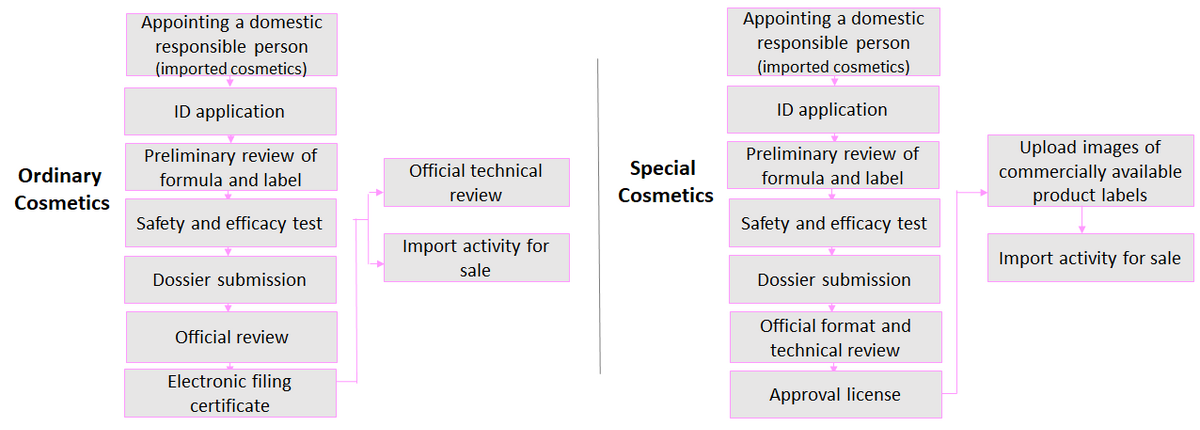
Registration and Filing Requirements
Materials required for cosmetic registration and filing
According to the Regulations on Cosmetic Registration and Filing Management, applicants must submit:
- Cosmetic Registration and Filing Information Form and supporting documents;
- Product name and formula;
- Standards implemented by the product;
- Draft product label;
- Product inspection report; and
- Product safety assessment data.
Testing requirements
All testing must comply with the Cosmetic Safety Technical Specifications, Regulations on Cosmetic Registration and Filing Inspection Work, and other relevant regulations, covering:
- Microbiological tests;
- Physicochemical tests;
- Toxicological tests;
- Human safety evaluation (for special cosmetics); and
- Human efficacy evaluation (for products with functional claims).
Toxicological test exemptions may apply when:
a) The manufacturer holds a valid GMP certificate issued by the competent authorities in their country or region.
b) A robust safety report confirms the safety.
Please note: If the product is produced by multiple manufacturers, each must have obtained GMP certification from the competent authorities in their country or region to qualify for an exemption from submitting toxicological test reports.
Exemptions do not apply to:
- Products for infants and children;
- Products containing new cosmetic ingredients that are still under safety monitoring; and
- Filers, domestic responsible persons, and manufacturers that are listed as key regulatory targets.
Cosmetic Labeling Requirements
According to the Cosmetic Label Management Measures, the Chinese label must include:
- Product name (in Chinese), and registration certificate number for special cosmetics;
- Name and address of the registrant/filer, and DRP (if applicable);
- The manufacturer's name and address, and the production license number (for domestic producers);
- Standard number implemented by the product;
- Full ingredient list;
- Net content and shelf life;
- Usage instructions and safety warnings; and
- Any other information required law or national standards.
If packaging includes an outer box, the Chinese product name and shelf life must also appear on the primary container that directly contacts the contents.
Post-Market Supervision
Ordinary Cosmetics
- The regulatory authorities conduct technical reviews and random sampling of filed cosmetics.
- Since 2022, all filed cosmetics must submit annual reports.
- Products identified with multiple safety or quality issues are subject to enhanced supervision or targeted inspections by drug regulatory departments.
Our Services
CIRS Testing provides full-cycle support for cosmetic registration and filing, helping brands achieve compliance efficiently and confidently.
Our services include:
- Acting as a Domestic Responsible Person (DRP);
- Determining the type of cosmetics (special vs ordinary) to put in place a compliance strategy;
- Preliminary review of formula and label;
- Safety and efficacy test coordination;
- Dossier preparation and submission; and
- Technical translation and regulatory communication.
Why Choose CIRS Testing?
- Advanced Laboratory Facilities: Fully equipped laboratories for phyicochemical, microbial, toxicological, and efficacy testing;
- Compliance Expertise: Extensive experience with Chinese regulatory standards and technical specifications;
- Technical Excellence: Highly skilled team of scientists and regulatory professionals;
- Global Service Network: Our global team can provide support for international brands exporting to China; and
- An Elite International Team: Multidisciplinary experts providing end-to-end testing and compliance services.
Our Expertise
The CIRS Testing Cosmetic Laboratory is accredited by the China National Accreditation Service for Conformity Assessment (CNAS), China Metrology Accreditation (CMA), and the National Medical Products Administration. These accreditations are not only essential for registration and filing of cosmetics in China, but they also demonstrate that our testing methods and data meet international testing and quality standards, ensuring that results are scientifically robust and globally recognized.
With a dedicated team of over 60 professionals, including experts in regulatory compliance, customer service, and laboratory research, we are committed to delivering accurate, compliant, and science-based testing solutions.
Our laboratory has developed multiple patents and maintains strong collaborations with national and international laboratories.
For expert support with cosmetic registration or filing in China, contact us at test@cirs-group.com.
