Overview
Disinfection products are designed to prevent disease by eliminating pathogenic microorganisms in the environment, not for treatment or diagnosis. These products work through chemical, physical, or biological methods, targeting environmental pathogens rather than targeting diseases in humans or animals.
In China, disinfection products include disinfectants, disinfection equipment (including biological indicators, chemical indicators, and packaging of sterilized items), sanitary products, and disposable medical supplies.
Disinfectant (Antibacterial/Bacteriostatic) testing items and standards
Product Category | Testing items | Testing standard | |
Disinfectant | Exterior | 1."Disinfection Technical Specifications 2002 Edition" 2.Corresponding national standards for various disinfectants 3.Enterprise standards for the record of each enterprise | |
Determination of active ingredient content | |||
PH measurement | |||
Stability test | |||
Continuous use stability test | |||
Determination of lead, arsenic and mercury | |||
Metal Corrosion Test | |||
Determination of the killing effect of microorganisms in the laboratory (Staphylococcus aureus, Escherichia coli, Candida albicans, Pseudomonas aeruginosa, Pseudomonas aeruginosa, Staphylococcus albicans, Aspergillus niger, M. virus, influenza virus, HIV, hand, foot and mouth virus, etc.) | |||
Simulate field trials or field trials (hard surfaces, hands, skin, air, medical devices, endoscopes, fabrics, food and drink utensils, etc.) | |||
Toxicological safety testing (acute oral toxicity test, acute inhalation toxicity test, one intact skin irritation test, multiple intact skin irritation test, eye irritation test, vaginal mucosal irritation test, one mutagenicity test, etc.) | |||
Overall performance test | |||
Antibacterial (bacteriostatic) agents /sanitary products | Determination of active ingredient content | 1. "Disinfection Technical Specifications 2002 Edition" 2. Hygiene Standard for Disposable Sanitary Products GB 15979-2002 3. Enterprise standards for the record of each enterprise | |
Stability test | |||
PH measurement | |||
Microbial indicators | The total number of bacterial colonies | ||
Coliform bacteria | |||
Total number of fungal colonies | |||
Pathogenic pyogenic bacteria | |||
Kill (inhibit) microbial indicators | E. coli kill (inhibition) test | ||
Staphylococcus aureus kill (inhibition) test | |||
Candida albicans kill (inhibition) test | |||
Other microbial killing (inhibition) tests | |||
Toxicological index detection | One complete skin irritation test, multiple complete skin irritation tests, eye irritation test, vaginal mucosal irritation test, acute oral toxicity test, etc. | ||
Corresponding national standards for various disinfectants
Types of disinfection products | Sanitation standard name and number |
Chlorine dioxide disinfectant | Hygienic standard for chlorine dioxide disinfectant GB 26366-2010 |
Guanidine disinfectants | Hygienic standard for guanidine disinfectants GB 26367-2020 |
Iodine-containing disinfectant | Hygienic standard for iodine-containing disinfectants GB 26368-2020 |
Quaternary ammonium salt disinfectant | Hygienic standard for quaternary ammonium salt disinfectants GB 26369-2020 |
Bromine-containing disinfectants | Hygienic standard for bromine-containing disinfectants GB 26370-2020 |
Peroxide-based disinfectants | Hygienic standard for peroxide-based disinfectants GB 26371-2020 |
Glutaraldehyde Disinfectant | Hygienic standard for glutaraldehyde disinfectant GB 26372-2020 |
Ethanol disinfectant | Hygienic standard for ethanol disinfectant GB 26373-2020 |
Phenolic disinfectants | Hygienic requirements for phenolic disinfectants GB 27947-2020 |
Air sanitizer | Hygienic requirements for air disinfectants GB 27948-2020 |
Hand sanitizer | Hand Sanitizer Hygiene Requirements GB 27950-2020 |
Skin disinfectant | Hygiene requirements for skin disinfectants GB 27951-2011 |
Disinfection of common surfaces | Sanitary requirements for surface disinfectants of ordinary objects GB 27952-2020 |
Foci disinfectant | Sanitary requirements for disinfectants in foci GB 27953-2020 |
Mucous membrane disinfectant | General requirements for mucosal disinfectants GB 27954-2020 |
Medical device disinfectant | Sanitary requirements for disinfectants for medical devices GB/T 27949-2020 |
Classification and management of disinfection products
Disinfection products in China are classified based on risk and use.
Category 1: High risk, requiring strict management to ensure safety and effectiveness. This covers high-level disinfectants and disinfection devices for medical devices, sterilants and sterilization devices, skin and mucous membrane disinfectants, biological indicators, and chemical indicators of sterilization effect.
Category 2: Moderate risk, management needs to be strengthened to ensure safety and effectiveness. This covers disinfectants, disinfection equipment, chemical indicators, and sterilized items other than Category 1 products, packaging with sterilization labels, and antibacterial (bacteriostatic) preparations.
Category 3: Low risk, and routine management can ensure safety and effectiveness. This covers sanitary products other than antibacterial (bacteriostatic) preparations.
Product responsible units must conduct sanitation and safety evaluations by themselves or by entrusting a third party before Category 1 and 2 disinfection products are put on the market for the first time, and shall be responsible for the evaluation results. Disinfection products that have passed the health and safety evaluation can be marketed.
Note: When the same disinfection product spans multiple categories, it should be managed by its highest risk category.
Legal basis for filing
- Administrative Measures for Disinfection
- Regulations on Sanitary Safety Evaluation of Disinfection Products
- Regulations on the Administration of Sanitation Administrative Licensing for New Disinfection Products and New Water-related Products
Filing materials generally include the disinfection product safety evaluation report, sanitation and safety evaluation report, labels, instructions, test reports, and other documents proving production and sales eligibility in China.
CIRS Testing provides disinfection product testing services but is not involved in the registration of new disinfection products.
Filing flow chart
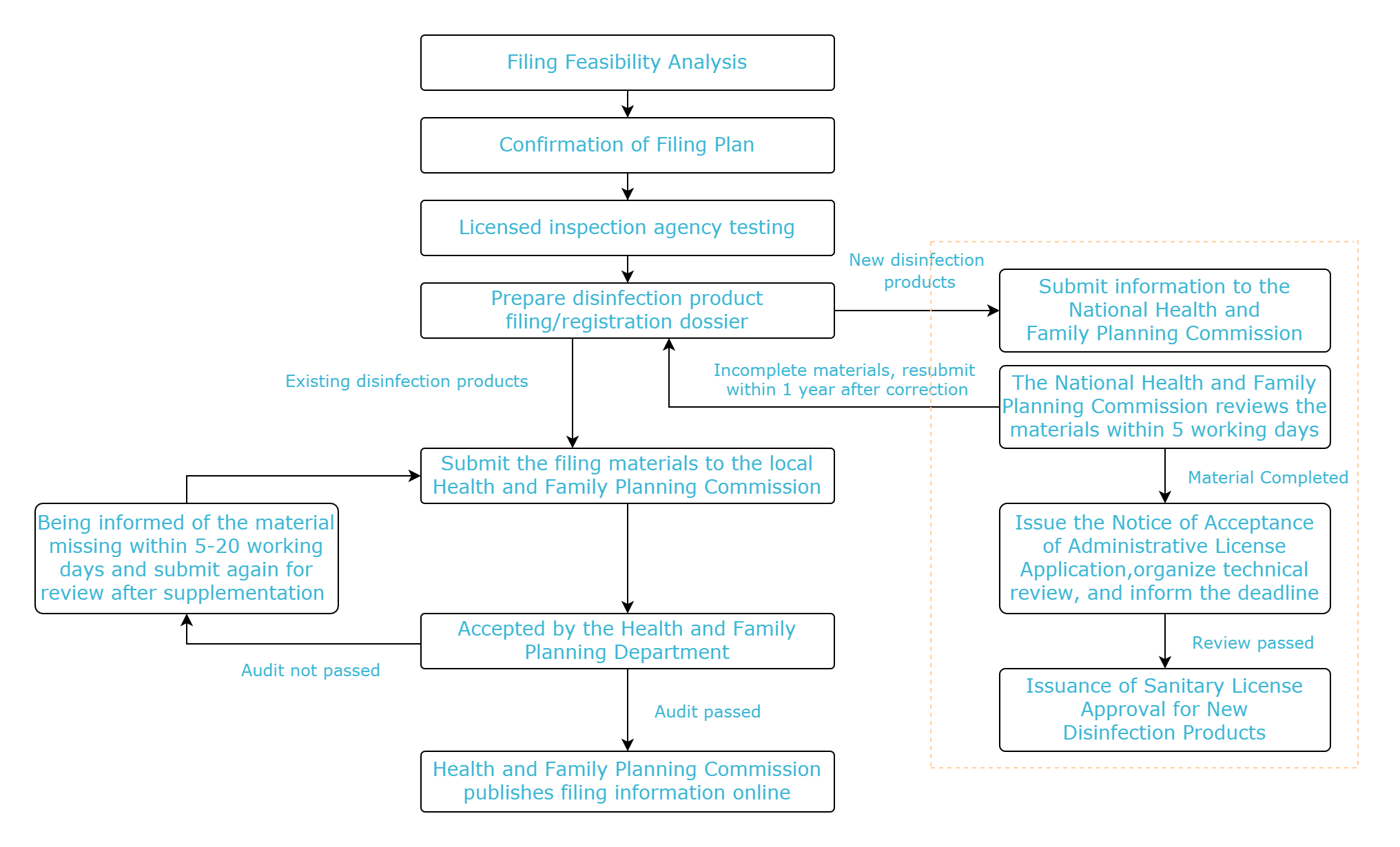
Contact CIRS Testing
CIRS Testing can support disinfection product companies with comprehensive testing services. For inquiries or testing requests, please contact us at test@cirs-group.com.

