21 June 2018, the Official Journal of European Union released a directive 2018/885, amending the Annex VI of the regulation (EC) 1223/2009 on cosmetics products. This directive shall be binding in its entirety and directly applicable in all Member States and enter into force on the twentieth day following that of its publication in the Official Journal of the European Union (i.e. 11 July 2018).

Annex VI to Regulation (EC) No 1223/2009 is amended as follows:
(1) Entry 23 is replaced by the following entry:
Reference
Number Substance identification Condition Chemical Name/INN/XAN Name of Common Ingredients Glossary CAS Number EC Number Maximum concentration in ready for use preparation 23 2,2’-Methylene- bis(6-(2H-benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenol)/Bisoctrizole Methylene Bis-Benzotriazolyl
Tetramethylbutylphenol 103597-45-1 403-800-1 10%
(2) Entry 23a is inserted:
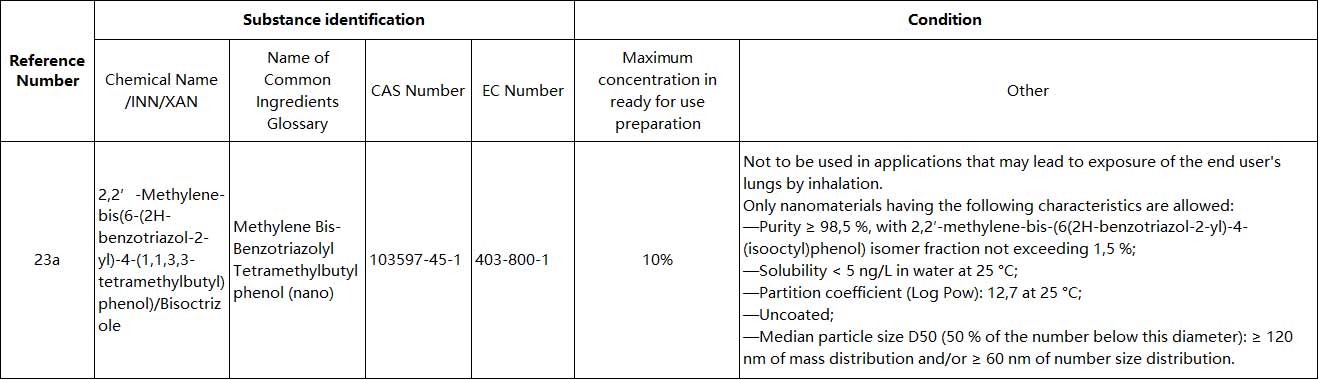
|Further Information:
OJ Notice of Amending the Regulation on Cosmetics Products
|Our Service:
