Definition of disinfection products
According to Article 78 of the Law of the People's Republic of China on the Prevention and Control of Infectious Diseases, disinfection refers to the use of chemical, physical and biological methods to kill or eliminate pathogenic microorganisms in the environment.
In terms of the purpose, this kind of products is used for disease prevention, but not for disease treatment or diagnosis; in terms of mechanism, it can eliminate pathogenic microorganisms by chemical, physical and biological methods, rather than pharmacological or immunological methods; in terms of the object of action, it mainly focused on pathogenic microorganisms in the environment rather than diseases.
According to Article 46 of the Measures for the Administration of Disinfection, disinfection products include disinfectants, disinfection equipment (including biological indicators, chemical indicators and packaging of sterilized items), sanitary products and disposable medical supplies.
Disinfectant (Antibacterial/Bacteriostatic) testing items and standards
Product Category | Testing items | Testing standard | |
Disinfectant | Exterior | 1."Disinfection Technical Specifications 2002 Edition" 2.Corresponding national standards for various disinfectants 3.Enterprise standards for the record of each enterprise | |
Determination of active ingredient content | |||
PH measurement | |||
Stability test | |||
Continuous use stability test | |||
Determination of lead, arsenic and mercury | |||
Metal Corrosion Test | |||
Determination of the killing effect of microorganisms in the laboratory (Staphylococcus aureus, Escherichia coli, Candida albicans, Pseudomonas aeruginosa, Pseudomonas aeruginosa, Staphylococcus albicans, Aspergillus niger, M. virus, influenza virus, HIV, hand, foot and mouth virus, etc.) | |||
Simulate field trials or field trials (hard surfaces, hands, skin, air, medical devices, endoscopes, fabrics, food and drink utensils, etc.) | |||
Toxicological safety testing (acute oral toxicity test, acute inhalation toxicity test, one intact skin irritation test, multiple intact skin irritation test, eye irritation test, vaginal mucosal irritation test, one mutagenicity test, etc.) | |||
Overall performance test | |||
Antibacterial (bacteriostatic) agents /sanitary products | Determination of active ingredient content | 1."Disinfection Technical Specifications 2002 Edition" 2.Hygiene Standard for Disposable Sanitary Products GB 15979-2002 3.Enterprise standards for the record of each enterprise | |
Stability test | |||
PH measurement | |||
Microbial indicators | The total number of bacterial colonies | ||
Coliform bacteria | |||
Total number of fungal colonies | |||
Pathogenic pyogenic bacteria | |||
Kill (inhibit) microbial indicators | E. coli kill (inhibition) test | ||
Staphylococcus aureus kill (inhibition) test | |||
Candida albicans kill (inhibition) test | |||
Other microbial killing (inhibition) tests | |||
Toxicological index detection | One complete skin irritation test, multiple complete skin irritation tests, eye irritation test, vaginal mucosal irritation test, acute oral toxicity test, etc. | ||
Corresponding national standards for various disinfectants
Types of disinfection products | Sanitation standard name and number |
Chlorine dioxide disinfectant | Hygienic standard for chlorine dioxide disinfectant GB 26366-2010 |
Guanidine disinfectants | Hygienic standard for guanidine disinfectants GB 26367-2020 |
Iodine-containing disinfectant | Hygienic standard for iodine-containing disinfectants GB 26368-2020 |
Quaternary ammonium salt disinfectant | Hygienic standard for quaternary ammonium salt disinfectants GB 26369-2020 |
Bromine-containing disinfectants | Hygienic standard for bromine-containing disinfectants GB 26370-2020 |
Peroxide-based disinfectants | Hygienic standard for peroxide-based disinfectants GB 26371-2020 |
Glutaraldehyde Disinfectant | Hygienic standard for glutaraldehyde disinfectant GB 26372-2020 |
Ethanol disinfectant | Hygienic standard for ethanol disinfectant GB 26373-2020 |
Phenolic disinfectants | Hygienic requirements for phenolic disinfectants GB 27947-2020 |
Air sanitizer | Hygienic requirements for air disinfectants GB 27948-2020 |
Hand sanitizer | Hand Sanitizer Hygiene Requirements GB 27950-2020 |
Skin disinfectant | Hygiene requirements for skin disinfectants GB 27951-2011 |
Disinfection of common surfaces | Sanitary requirements for surface disinfectants of ordinary objects GB 27952-2020 |
Foci disinfectant | Sanitary requirements for disinfectants in foci GB 27953-2020 |
Mucous membrane disinfectant | General requirements for mucosal disinfectants GB 27954-2020 |
Medical device disinfectant | Sanitary requirements for disinfectants for medical devices GB/T 27949-2020 |
Classification and management of disinfection products
China implements classified management of disinfection products according to the use and the risk level of the object of use. Product responsible units shall conduct sanitation and safety evaluations by themselves or by entrusting a third party before the Category 1 and Category 2 disinfection products are put on the market for the first time, and shall be responsible for the evaluation results. Disinfection products that have passed the health and safety evaluation can be marketed.
Category 1: High risk, requiring strict management to ensure safety and effectiveness. This kind of disinfection products covers high-level disinfectants and disinfection devices for medical devices, sterilants and sterilization devices, skin and mucous membrane disinfectants, biological Indicator, chemical indicator of sterilization effect.
Category 2: Moderate risk, management needs to be strengthened to ensure safety and effectiveness. This kind of disinfection products covers disinfectants, disinfection equipment, chemical indicators, and sterilized items other than Category 1 products, packaging with sterilization labels, antibacterial (bacteriostatic) preparations.
Category 3: Low risk, and routine management can ensure safety and effectiveness. This kind of disinfection products covers sanitary products other than antibacterial (bacteriostatic) preparations.
Note: When the same disinfection product involves different categories, it should be managed by its highest risk category.
Legal basis for filing
- Administrative Measures for Disinfection
- Regulations on Sanitary Safety Evaluation of Disinfection Products
- Regulations on the Administration of Sanitation Administrative Licensing for New Disinfection Products and New Water-related Products
Specific information requirements
Disinfection product filing materials are generally the filing registration form of the disinfection product safety evaluation report, the disinfection product sanitation and safety evaluation report, labels, instructions, test reports and other basic materials. It is necessary to have documents and other materials that allow the production and sales of imported products in the country (region).
CIRS is not involved in the registration of new disinfection products.
Competent authority: provincial (city, district) health and family planning committees
Filing flow chart
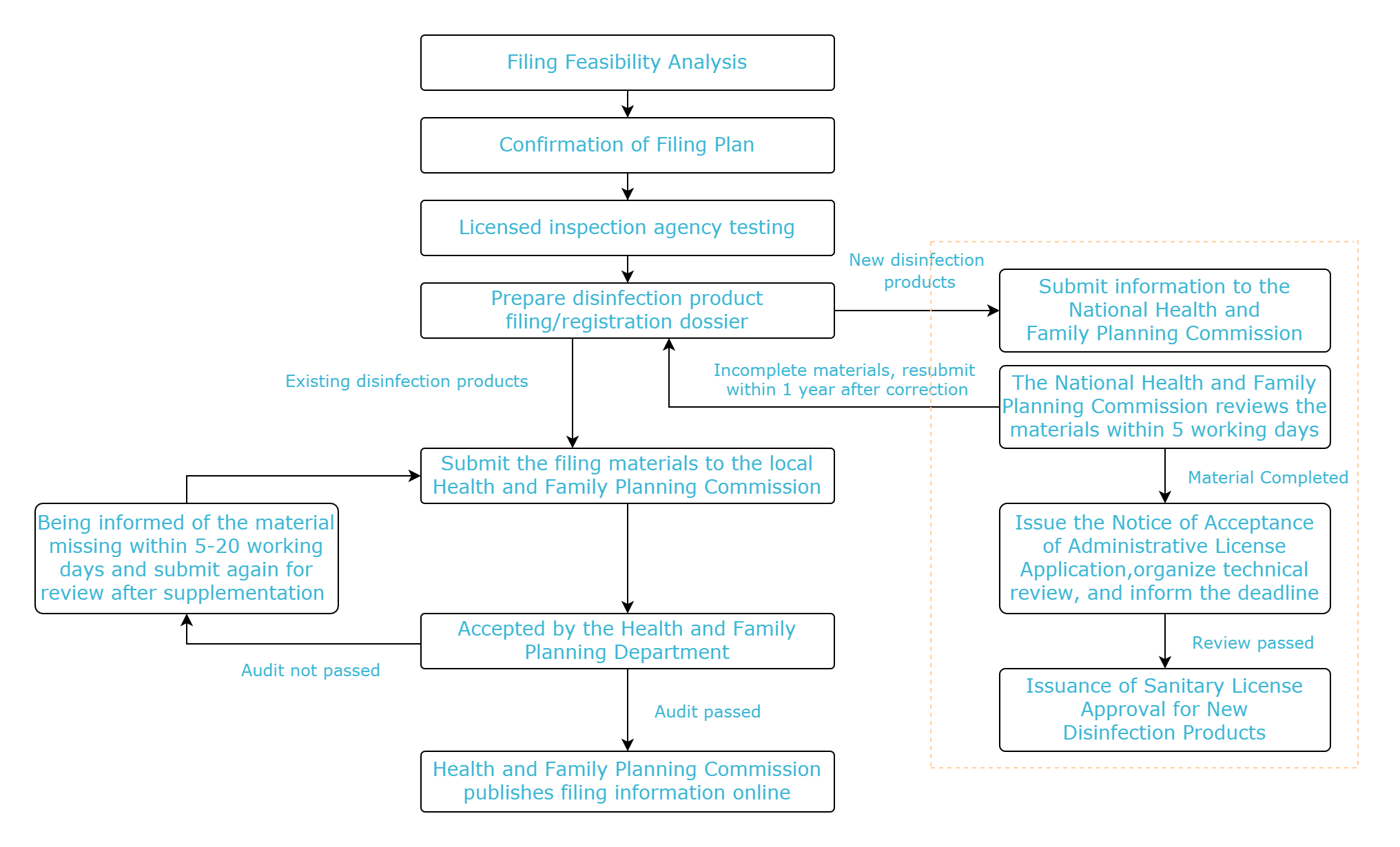
C&K Testing can provide testing services for the majority of disinfection product companies. If you have any needs or questions, please contact us at test@cirs-group.com.
China Disinfectant Notification Testing

